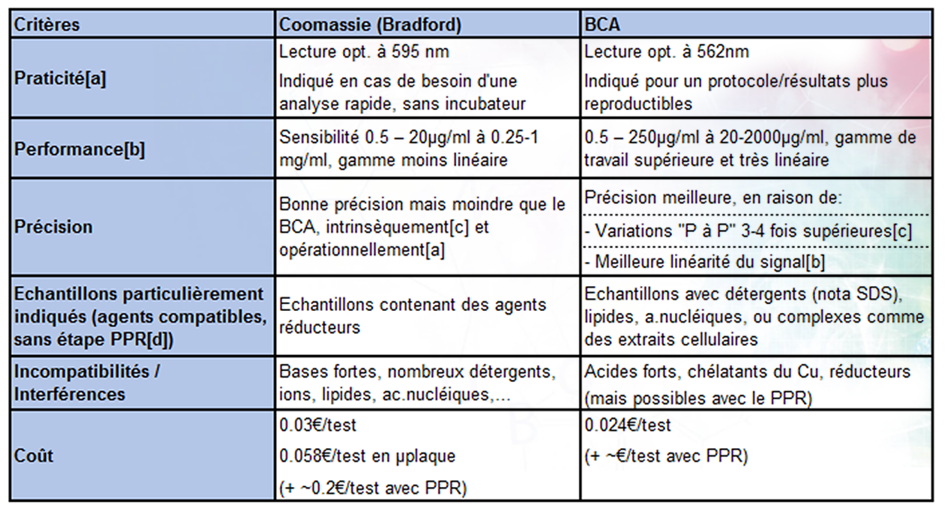
Biuret methods are widely used in biochemistry to detect peptide bonds and the presence of proteins. They offer ease of use and high sensitivity, enabling precise detection of protein concentrations. However, they can have drawbacks such as interference with other compounds and low specificity. Despite these limitations, the biuret method remains a key tool in protein analysis.
10 - Bradford Assay
[arve url="https://www.youtube.com/embed/EhinSM9FZCg "/]
What is the significance of the biuret reaction?
The biuret reaction is a commonly used method for detecting and quantifying proteins in biological samples. It is based on the formation of a colored complex between the peptide bonds of proteins and the biuret reagent.
The importance of the biuret reaction in the context of a news site lies in its relevance to the coverage of topics related to science, medicine and biology. Numerous articles and features can be devoted to scientific advances, medical discoveries or biological research involving the study and characterization of proteins.
Using the biuret reaction, it is possible to perform qualitative or quantitative tests to determine the presence and concentration of proteins in a variety of situations. For example, this could include news about developments in medical diagnostics, disease research or diet and nutrition studies.
In addition, the biuret reaction can be a valuable tool for assessing the efficacy of biomolecular therapies, the quality of food products or the environmental impact of chemical substances on living organisms.
Finally, it's important to stress that the biuret reaction is just one of many methods available for protein analysis. However, its simplicity, speed and sensitivity make it a widely used and recognized technique in the scientific community.
In short, the biuret reaction is a crucial technique for detecting and quantifying proteins, and it finds an important place in the context of a news site focused on science, medicine and biology.
How does the Biuret method work?
The Biuret method is a chemical technique used to detect the presence of proteins in a sample. It is based on the fact that peptide bonds, present in proteins, react with Biuret solution to form a characteristic violet complex.
The principle of the Biuret method is as follows:
1. Sample preparation : The protein-containing sample is prepared by denaturing it, i.e. breaking down the secondary and tertiary structures of the proteins to expose the peptide bonds.
2. Reaction with Biuret solution: The sample is then mixed with a solution containing copper(II) sulfate and sodium tartrate. The copper (II) ions present in the solution react with the peptide bonds, forming a violet complex.
3. Absorbance measurement : The formation of the violet complex is measured using a spectrophotometer. The absorbance of the solution is directly proportional to the concentration of protein in the sample.
The advantages of this method :
The Biuret method is commonly used in biochemistry to quantify the concentration of proteins in a sample. It is simple, fast and relatively accurate. What's more, it can be used to detect various types of protein, making it a versatile method.
It's important to note that this method doesn't specifically identify which proteins are present in the sample, it simply indicates the total protein concentration. For specific protein identification, other techniques, such as electrophoresis, are required.
Why does the biuret test reveal proteins?
The biuret test is used to detect the presence of protides, or more precisely proteins, in a sample. The test is based on the chemical reaction between proteins and biuret reagent.
Proteins are macromolecules composed of amino acids linked by peptide bonds. The biuret reagent is an alkaline copper(II) sulfate solution containing sodium hydroxide. When proteins react with biuret reagent, the peptide bonds are broken, leading to the formation of copper-protein complexes.
These copper-protein complexes have a characteristic violet color, visualizing the presence of proteins in the sample under test. The more proteins in the sample, the more intense the violet color. This is why the biuret test is used to detect the presence of protides, or proteins, in samples.
This test is commonly used in many fields, such as biology, biochemistry and medicine, to determine the presence and quantity of proteins in various biological samples. It is also used in the food industry to control the quality of protein-based products, such as meats, dairy products and meat substitutes.
What method is frequently used to estimate the concentration of a protein in solution?
The most commonly used method for estimating the concentration of a protein in solution is spectrophotometry. This technique is based on the fact that proteins absorb light at specific wavelengths. By measuring the absorbance of the protein solution at a certain wavelength, the concentration of the protein can be determined.
To apply this method, it is necessary to know the molecular extinction coefficient (ε) of the protein under study. This coefficient depends on the protein's amino acid sequence, and is generally supplied by the manufacturers. Using Beer-Lambert's law, which establishes a linear relationship between absorbance, concentration and molecular extinction coefficient, it is possible to calculate the concentration of the protein in solution.
It should be noted that spectrophotometry is an indirect method which does not directly measure the amount of protein in solution, but rather the absorption of light by the protein. Consequently, it is recommended to use this method in combination with other techniques, such as gel electrophoresis or chromatography, to obtain a more accurate estimate of protein concentration.
In conclusion, spectrophotometry is a widely used method for estimating the concentration of proteins in solution, but its accuracy depends on knowledge of the molecular extinction coefficient specific to the protein under study.
In conclusion, the biuret method has both advantages and disadvantages in protein analysis. On the one hand, this method is relatively simple to implement, and offers fast, accurate results thanks to its specificity for peptide bonds. What's more, it can be used to quantify proteins present in large quantities.
On the other hand, the biuret method has a number of limitations. It cannot differentiate between different types of protein, and gives no information on their structure or function. What's more, this method requires a large quantity of sample, which can be a drawback when resources are limited.
In conclusion, It is important to consider the advantages and disadvantages of the biuret method before using it in a protein analysis. It may be wise to combine this method with other techniques to obtain more complete information on the proteins under study.