Bulk polymerization is a process commonly used in industry to create polymers on a large scale. However, it has both advantages and disadvantages. On the one hand, it enables polymers to be produced quickly and economically. However, it can be difficult to precisely control the properties of the final product and avoid the formation of imperfections. It is therefore important to weigh up these advantages and disadvantages before using bulk polymerization in a manufacturing process.
Radical polymerization
[arve url="https://www.youtube.com/embed/HiEzlDLlcu4″/]
What are the properties of polymers?
Polymers are materials composed of long chains of repeating molecules called monomers. These molecules can be natural, like DNA and proteins, or synthetic, like plastic and rubber.
The properties of polymers depend on their chemical structure and molecular arrangement. Here are some of the most important properties:
- Mechanical strength : Polymers can be very strong and have a high load-bearing capacity. This makes them useful in many applications, from construction to sports equipment.
- Flexibility : Polymers can be flexible and elastic, allowing them to bend without breaking. This is why they are often used in the manufacture of products such as gaskets and flexible packaging.
- Waterproofing : Some polymers are impermeable to water and gases, making them ideal for coatings and waterproofing membranes.
- Electrical insulation : Many polymers have low electrical conductivity, making them ideal insulating materials for cables and electronic components.
- Chemical resistance : Some polymers are resistant to chemicals, making them suitable for storing corrosive or hazardous substances.
- Lightweight : Polymers can be very light, making them useful in applications where weight is an important factor, such as the aerospace industry.
- Thermal stability : Some polymers are stable at high temperatures, making them suitable for use in hot or heat-exposed environments.
Thanks to their wide range of properties, polymers are widely used in many fields, including industry, medicine, electronics and packaging.
What are the three main classes of polymers according to their properties?
The three main classes of polymers according to their properties are as follows:
1. Visit thermoplastic polymers : These are polymers that can be softened and melted at high temperatures, then cooled to harden again. They retain this ability to soften and harden several times. Thermoplastic polymers are widely used in a variety of applications, including packaging, automotive and electronics.
2. The thermosetting polymers : Unlike thermoplastic polymers, thermoset polymers cannot be softened and remelted after curing. They undergo a chemical reaction during curing, making them permanently rigid. Thermoset polymers are often used in applications requiring high dimensional stability and heat resistance, such as composites, coatings and adhesives.
3. Visit elastomers : Elastomers are polymers with the ability to return to their original shape after being stretched or deformed. They possess significant elastic properties, making them ideal for applications such as seals, tires and damping materials.
It's important to note that each class of polymer has its own unique characteristics in terms of mechanical strength, flexibility, chemical resistance, etc., making them suitable for specific uses in different industrial sectors.
What is an example polymerization reaction?
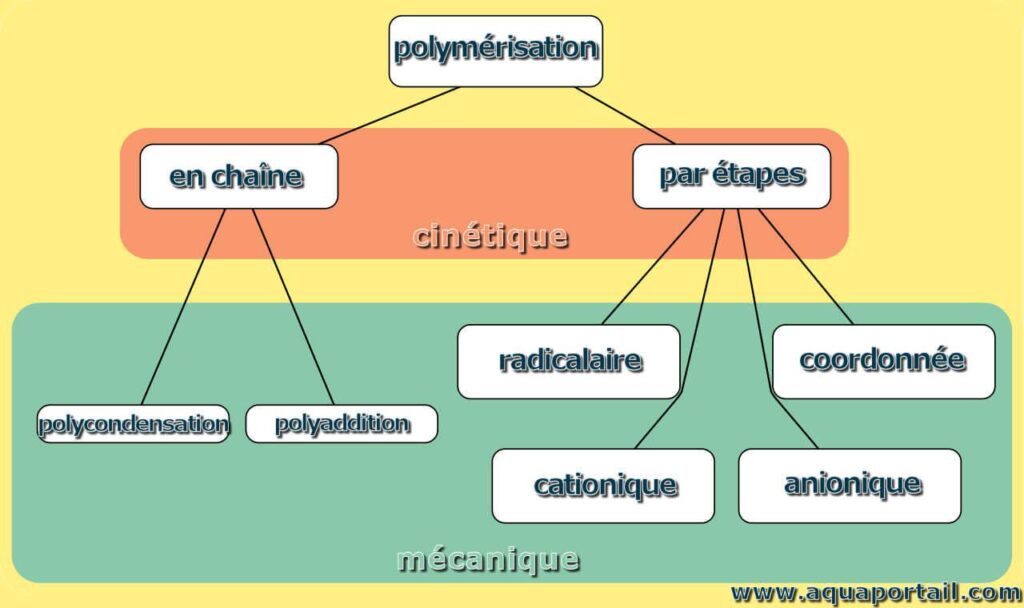
A polymerization reaction is a chemical process in which monomers bond together to form a polymer. There are different types of polymerization reactions, of which the following are a few examples:
1. Addition polymerization : This is a reaction in which the double bonds of unsaturated monomers are broken to form covalent bonds with other monomers. A common example is the polymerization of propylene to form polypropylene, which is used in the manufacture of various plastic and textile products.
2. Condensation polymerization : is a reaction in which two monomers react by eliminating a small molecule, such as water or another condensation product. A well-known example is the polymerization of ethylene glycol and terephthalic acid to form polyester, used in the manufacture of textile fibers and plastic bottles.
3. Chain polymerization : This type of reaction involves a succession of chain reactions, where monomers react with initiating agents to form polymer chains. A popular example is the polymerization of styrene to obtain polystyrene, widely used in the food and packaging industries.
In a nutshell, The polymerization reaction is a key phenomenon in the formation of polymers from monomers. These examples of different polymerization reactions illustrate how molecules can be transformed to create a wide variety of materials used in our everyday lives.
Why polymerization?
Polymerization is a key process in many scientific and industrial fields. It consists in the chemical reaction that links several molecules called monomers to form a large macromolecular structure called a polymer.
Polymerization has many practical applications. In the plastics industry, for example, it enables the production of strong, flexible and durable materials. The resulting polymers can be used to manufacture plastic bottles, toys, stretch film, etc.
Polymerization is also used in the coatings industry. Polymer coatings offer protection against corrosion, scratches and weathering. They are used in the painting of cars, buildings and other metal surfaces to protect them and improve their appearance.
Polymerization also plays an important role in medicine and pharmacology. For example, it can be used to manufacture biocompatible materials for medical implants, controlled-release drugs and cell culture media.
In conclusion, polymerization is a fundamental process with numerous applications in various sectors. It contributes to the manufacture of more durable, resistant and functional products, and plays an essential role in the advancement of science and technology.
In conclusion, mass polymerization has both advantages and disadvantages. On the one hand, this polymerization method is fast, efficient and economical, enabling large quantities of polymer to be produced in a short time. In addition, it offers excellent material homogeneity and great flexibility in the choice of monomers.
On the other hand, mass polymerization can give rise to problems of temperature control and air bubble formation, which can lead to quality defects. In addition, it can be difficult to control bulk polymerization to obtain specific properties of the final polymer.
Despite these disadvantages, mass polymerization remains a widely used method in industry due to its speed and efficiency. However, the necessary polymerization conditions must be taken into account to avoid any potential problems.
In short, mass polymerization is a versatile technique, but one that requires particular care when it comes to implementation. It offers undeniable advantages in terms of mass production, but requires awareness of the limitations inherent in the process.